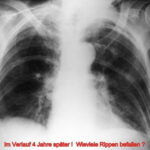
14.11.25/16.12.25
1) The “overview X-ray” cannot be used as a screening method for bone metastases (BM), even under optimized technical and training conditions.
This fact is deepened and supported by an autopsy study.
2. Although not intended for BM screening, X-ray examinations are conducted for other diagnostic purposes. Therefore, it is important to enhance diagnostic accuracy to avoid missing “incidental findings,” i.e., existing X-ray findings.
What makes the already very challenging to nearly impossible diagnosis even more difficult?
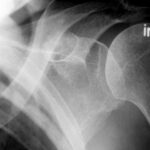
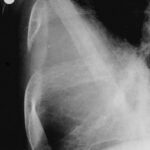
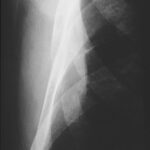
- Lack of experience of the examiner delays diagnosis (and thus worsens the therapy).
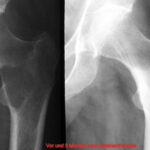
- Technical details also play a role, e.g., underexposure, screen settings too bright
- Unfavorable projection
- Overlap
- Artifacts
- Missing side-by-side comparison and
- Absence of prior examinations.
Even if these parameters are improved, summation radiography as a screening method for bone metastases (BM) remains obsolete!
More sensitive and specific methods such as MRI, skeletal scintigraphy, and biopsy are available, with additional support from CT.
Bone metastases develop from tumor cell deposits in the marrow cavity (“osteoneutral” BM). Radiographic detectability only begins with involvement of bone tissue and a significant imbalance between osteoblasts and osteoclasts.
In addition to the lack of representation of the marrow cavity and bone metabolism, there are other reasons for the low sensitivity of native examination:
- Inaccuracies in standard settings and exposure
- Overlap in large skeletal sections (e.g., spine and central pelvis),
- Lack of knowledge about typical variants, preferred regions for BM, and technical sources of error,
- Finally, misdiagnoses further reduce the already low sensitivity.
The weaknesses of native diagnostics should not lead to systematic oversight of bone metastases.
A. Autopsy Study
The overview image shows only the tip of the iceberg in terms of BM.
Material and Method:
Two series of 50 + 32 = 82 autopsy-derived sternum specimens.
The study examined how many macroscopic bone metastases can be visualized using X-ray techniques. The comparability of methods is initially complicated in the sternum by different habits in projection; sagittal saw cuts are common in specimen preparation. The coronal saw cut developed by the author improves comparability (Fig.1).
Overview radiograms were performed using conventional film technique: 200-speed intensifying screens (Fig.3a) at 50 kV, and additional conventional mammography technique (32 kV; Figs. 2 and 3).
In 50 specimens, direct coronal CT slices were performed (Fig.3b). This method was developed and standardized for this study.
Results:
Of the 336 macroscopic metastases identified, only 139 (= 41.4%) were detected using X-ray techniques.
Even under these technically optimized conditions (with specimens available and no overlapping structures), the proportion of undetected metastases was higher than the 50% reported in the literature.
Discussion:
The two conventional techniques were equally limited in their diagnostic value. (If microscopic metastases, only detectable under a microscope, were also counted, the results for X-ray methods would be even worse).
The (native) CT demonstrated numerous incidental findings in this study setup but did not detect significantly more metastases (Fig.3b). The situation is different in regions with extensive overlap, such as the axial skeleton, pelvis, and skull in living patients; here, the CT demonstrates its advantages as a sectional imaging technique.
B. Summary of Pathology, X-ray Diagnosis, and Didactic Notes
Variability in the Pathology and Clinical Presentation of Bone Metastases (BM).
Not only the imaging (see X-ray classifications), but also the clinical presentation varies significantly.
On one hand, the clinical picture may include severe, difficult-to-manage pain, while on the other hand, there may be clinically silent metastases discovered as incidental radiological findings (Fig.14) or surprise findings during autopsy (Fig.4). (In the past, these were estimated at 50%; today, the frequency of surprises has decreased significantly with modern techniques).
(Special issues arise with metastases that are detected before the primary tumor is found).
Frequency of Primary Tumors Leading to Bone Metastases:
Bone metastases from breast, prostate, and bronchial carcinoma account for 80% of all BM cases.
Pathways of Bone Metastasis Development:
Only rarely does direct infiltration by malignant tumors occur; instead, the following pathways of metastasis development predominate:
- Hematogenous spread,
- Portal vein pathway,
- Pulmonary vein, e.g., in bronchial carcinoma; common pathway for BM.
- Vena cava, lung passage, systemic circulation, e.g., breast and prostate carcinoma; common pathway for BM.
The latter three are rather rare pathways for BM development.
An important pathway for BM in prostate carcinoma: From the vena cava to the Batson’s venous plexus and the rectal venous plexus (Fig.7; 7a). –
In other tumors, the preferred skeletal region can be approximated according to the distribution of red bone marrow (age-dependent regression). In the case of anemia during advanced metastasis, the likelihood of preference for the axial skeleton increases further.
Radiological Type of Bone Metastasis Depending on Histological Growth Pattern:
- “Solid” (= largely synonymous with “medullary”): Predominantly shows an “osteopenic” appearance (Fig.5; 5b; 14; 14a; 17).
- “Cirrhotic”: Predominantly shows “osteosclerotic” type (Fig.4; 7; 7a; 8; 15; 15b).
- “Adenomatous”: Predominantly shows “mixed-type metastases” (Fig.11b; 16).
Individual Distribution of Bone Metastases:
- Solitary (Fig. 5b); modern methods show that such BM are even rarer than previously thought,
- Oligotopic
- Generalized
C. Simplified and Detailed Classifications of Bone Metastases (BM)
2.1. Classification of BM by Their Location in the Bone and Its Impact on Diagnostic Errors
- Central (Fig.2; 6; 17)
- Cortical involvement (Fig.5; 5b; 10; 11; 14; 14a)
- Periosteal reaction (Fig.16a; 16b)
- Pathological fractures (“callus formation” as a sign of repair can contribute to the appearance of bone metastases).
This is a simplified classification, useful for sorting diagnostic errors:
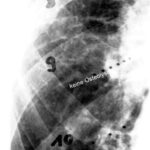
Compared to the difficult-to-diagnose central osteolysis with fine trabecular blurring (Fig.2), the cortical lysis (Fig.10) is a very clear sign. Nevertheless, it is still often overlooked.
The “periosteal reaction” is also a neglected finding. The possibility of malignant activity is frequently not considered (Fig.16a; 16b).
2.2. Lodwick Classification of BM
The “Lodwick Classification” can also be applied to secondary bone tumors. The diversity of appearances results in a variety of Lodwick grades: Ic, II, and III. Despite the different classifications, a high likelihood of malignancy is evident in this scheme.
2.3. Carcinomatous Dysplasia According to Burkhardt (1982)
The classification derived by Burkhardt from iliac crest biopsies shows a high agreement with radiological classifications. The author has significantly contributed to understanding BM as a complex pathological process through detailed analyses: There are humoral substances that play a mediating role in bone resorption and formation. The radiologically visible process can be driven by a chemically mediated process without detectable tumor cells.
Among the destructive changes, Burkhardt distinguishes:
- Moth-eaten (= lacunar = permeative) destruction
- Substance-destructive fragmentation
- Diffuse osteopenia as a typical metastatic bone change
- In periosteal bone formation, the radiomorphological distinction between carcinomatous and nonspecific paraneoplastic apposition is not clear.
2.4. Classification of Bone Metastases by Radiological Characteristics (Freischmidt)
– 2.4.1. For lytic BM, the following are distinguished:
- Typically osteolytic changes and
- “Cystic” expansive forms.
In the latter, the bone is expanded with a space-occupying lesion: A soft tissue tumor extending beyond the bone cortex or leaving a thin periosteal shell (Fig. 16a). (Differential diagnosis: Solitary plasmacytoma or aneurysmal bone cyst).
– 2.4.2. Osteoblastic Metastases are:
- Partially uniform,
- Partially very patchy (Fig. 7).
- Transitions from patchy to confluent osteoblastic structures occur (Fig. 7a).
(Differential diagnosis: Paget’s disease is characterized by unilaterality and restriction to pelvic bones).
– 2.4.3. Mixed-type BM, as seen in breast, stomach, and thyroid carcinoma (Fig. 11b; 15b; 16), present the following challenges:
- Distinguishing osteoblastic metastases from reactive or reparative osteosclerosis.
- Separating the lytic components from normal bone between osteoblastic lesions.
- Periosteal changes (tumorous or reactive) are most commonly seen in small-cell bronchial carcinoma (Fig. 16b).
A distinct form is the hypertrophic osteoarthropathy of the tubular bones typical of bronchial carcinoma (syndromic with clubbed fingers). Here, there is no evidence of tumor deposits.
This covers four common classifications, and now we proceed to:
Primary Tumors and Their Likely Radiological Findings in BM:
- Small-cell bronchial carcinoma: Permeative, moth-eaten appearance
- Adenocarcinoma and squamous cell carcinoma of the lung: Expansive lesions
- Breast carcinoma: Half are mixed-type; episodic progression with phases of remission
- Prostate carcinoma: Osteoblastic, with a preference for the pelvis and spine
- Renal cell carcinoma: Lytic (and highly vascularized)
- Thyroid carcinoma: Predominantly lytic with an expansive component; often “mixed-type” in widespread cases
- Gastric carcinoma: Rare, predominantly osteoblastic
- Cervical carcinoma: Rare, predominantly mixed-type
- Ovarian, testicular, gallbladder, pancreatic, liver, and colon carcinoma: Due to their rarity, little reliable statistical data on metastatic character is available
D. Imaging. Exanples; Section 1
If the images appear too dark, please temporarily adjust the screen brightness! The black background is useful.
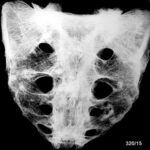
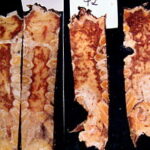
Fig. 1: Four sternum specimens split by sagittal saw cuts and opened. Macroscopically, the red marrow is displaced by multiple metastatic lesions.
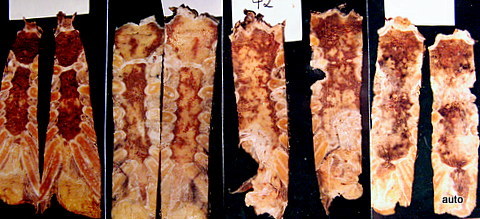
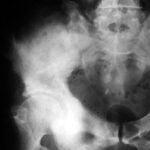
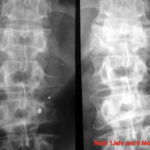
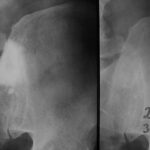
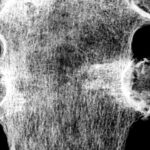
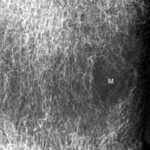
Fig. 2: Radiogram with a (M) larger lesion showing rarefied trabecular structure. In addition to this clearly lytic lesion, other smaller suspicious areas are present.
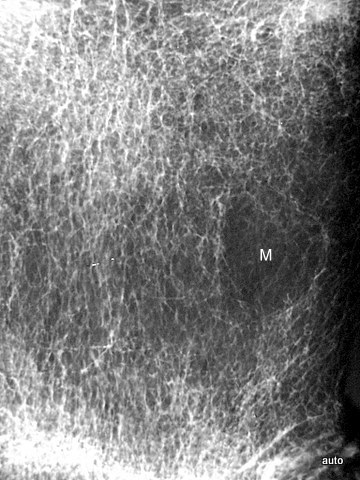
Fig. 3: Multiple smaller, partly lytic and partly osteoblastic lesions. Only rarely are the macroscopically confirmed metastases as clearly visible in the overview X-ray image.
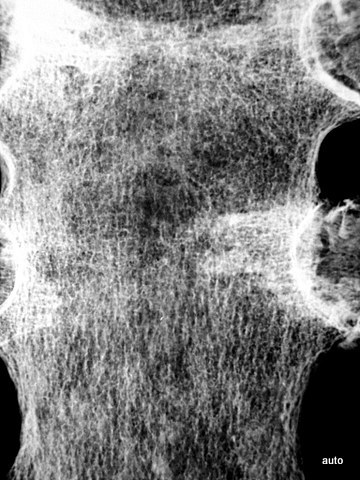
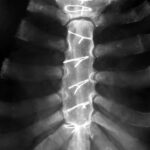
Fig. 3a: Radiogram without suspicion of metastasis (incidental finding: wire cerclages after sternotomy and consolidation).
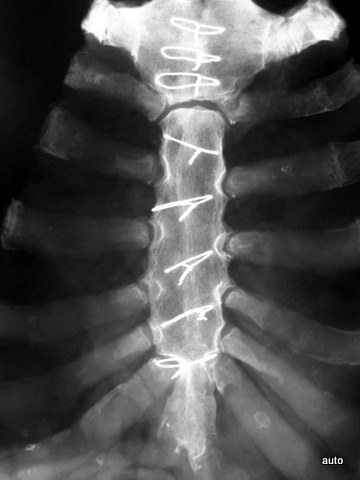
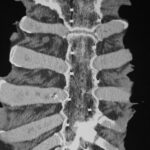
Fig. 3b: Corresponding coronal computed tomography (CT) slice. We deal with some artefacts.
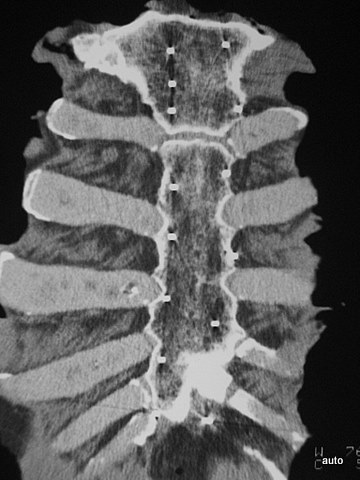
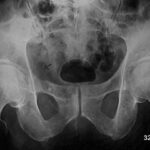
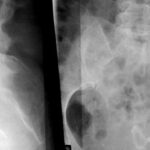
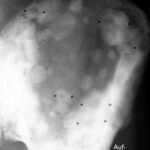
Fig. 4: Despite shortcomings, the overview radiography can still be effective: Sacral specimen from a patient with advanced prostate carcinoma (death from heart disease, not from progressive tumor). The surface of the sacrum appeared normal, but the X-ray showed multiple small osteoblastic lesions.
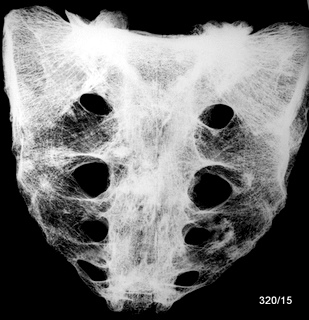
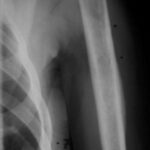
Fig. 5: Extensive lysis due to bone metastasis in the upper scapula, including large parts of the acromion in a bladder carcinoma case. Large-scale destruction of the cortex. The existing soft tissue tumor (see also Figs. 14; 14a) is not visible here.
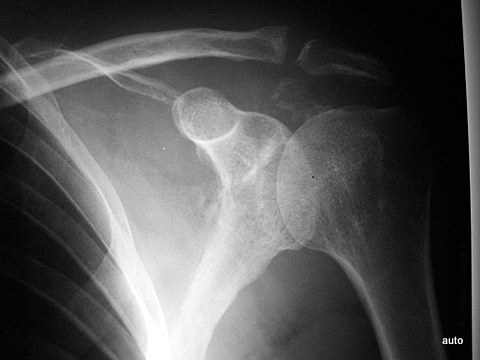
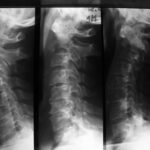
Fig. 5a: Dislocation and subluxation must also be considered in cases of unclear clinical symptoms, similar to fractures. Lateral cervical spine; initial image on the far left, middle and right show follow-ups after 1 and 4 months. Progressive displacement of the anterior atlas and axis due to metastatic destruction, visible by the marked posterior edge of the axis and the displacement of the posterior arch sections of C1 and C2.
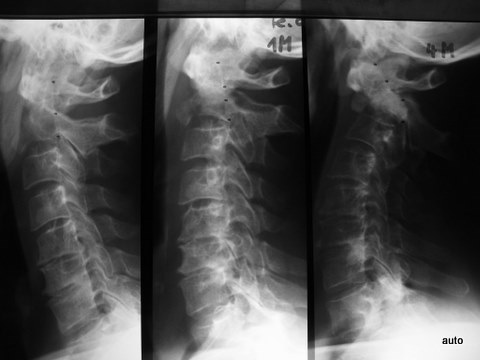
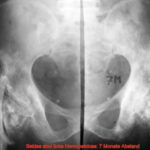
Fig. 5b: Changes due to callus formation can also contribute to the appearance of bone metastases: Osteolysis of the clavicle involving the cortex. Pathological fracture and formation of reparative tissue. (Follow-up after 1 and 3 months)
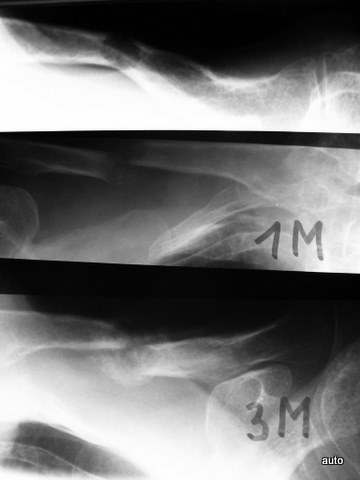
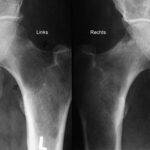
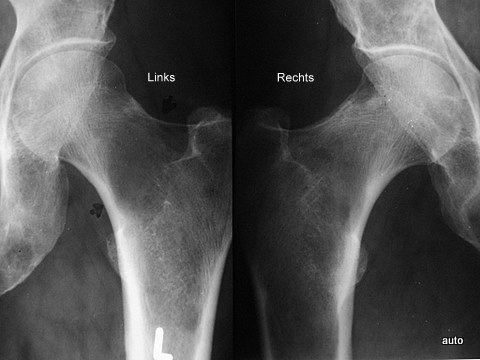
Fig. 6: Comparison of both femurs made easier by close juxtaposition: Large lysis in the left femoral neck; it is not easily recognizable due to the naturally low trabecular density in this region. Only in the comparison does it become clear that the rarefaction of the bone structure exceeds physiological levels. Several smaller lyses on the right; slight periosteal reaction on the upper contour of the femoral neck.
4. Imaging Section 2
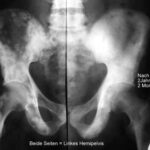
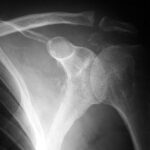
Fig. 7: Unusual osteoblastic lesions in prostate carcinoma with central radiolucencies; likely therapy effect.
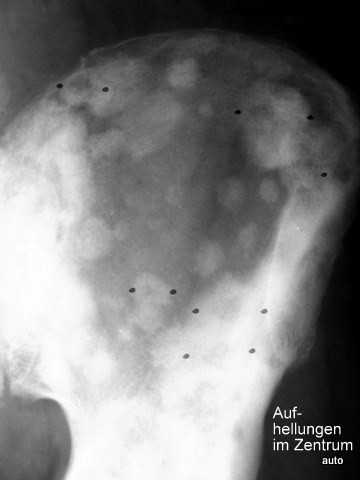
Fig. 7a: Same case. Follow-up after 2 years and 2 months. Both left pelvic halves are symmetrically aligned! The difference is the time; both sides are the same. Transition from patchy lesions to a confluent osteoblastic structure. Disappearance of the peculiar central radiolucencies. The lesions in both femurs appeared later.
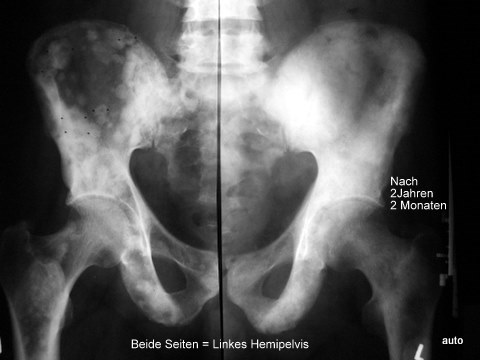
Fig. 8: New case of osteoblastic metastases in prostate carcinoma. (Such BM usually cause less pain than lytic lesions). Here, however, there was severe pain (Left pic.). After radiation and hormone therapy (Right pic.): Significant clinical improvement and regression of osteoblastic BM, even after 2 years and 3 months. At this point, a description of a normal finding would not have been accurate.
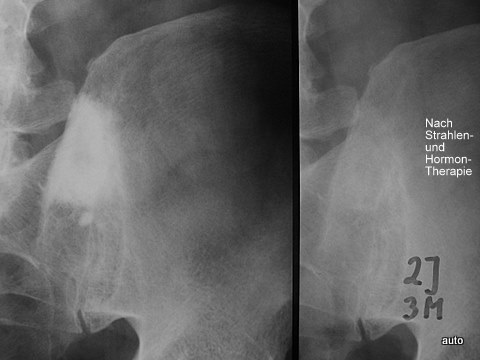
Fig. 9: Model for the cortical bone. The pages of a book are radiologically depicted when sufficient “mass” is summed through tangential projection. Similarly, the cortical bone is well visualized tangentially, revealing defects. In orthogonal views, visualization is much poorer or even absent. This is demonstrated with examples of vertebral arches, foramina, and ribs.

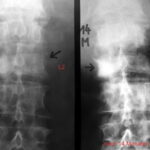
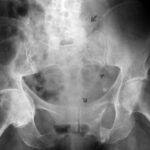
Fig. 10: Breast carcinoma diagnosed 5 years ago. Severe lower back pain now. Large (fist-sized) osteolysis in the right sacrum. The crucial finding is the obliteration of the right sacral foramina! Overlapping bowel content could degrade contrast but does not explain the obliteration of these structures.
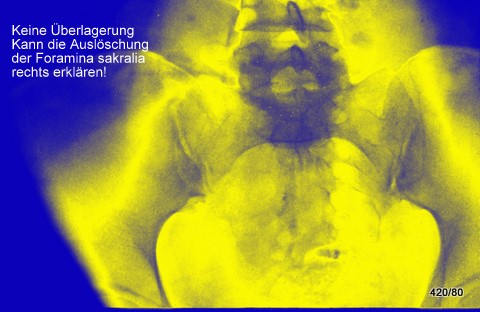
In a test, only 4 out of 200 first-year medical professionals recognized this finding!
No overlap (e.g., bowel gas) can explain the obliteration of the right foramina!
Fig. 10a: Breast carcinoma. Initial diagnosis 4 years ago. Repetition of radiographic signs from Fig. 10. Loss of structures in individual nerve exit holes: L5 left, sacrum left (arrows). Sonographically, a large soft tissue tumor. Fractures, likely pathological, in the anterior pelvic ring left (arrow). Incidental finding: Coxarthrosis on the right.
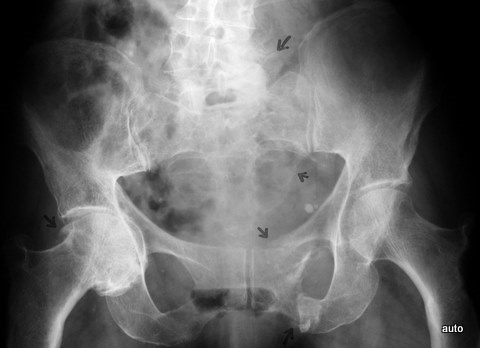
Fig. 10b: Status post-surgery of rectal carcinoma. Pain. Destruction of the lower sacrum. The lateral projection is also important; the upper parts of the anterior sacral wall are still preserved.
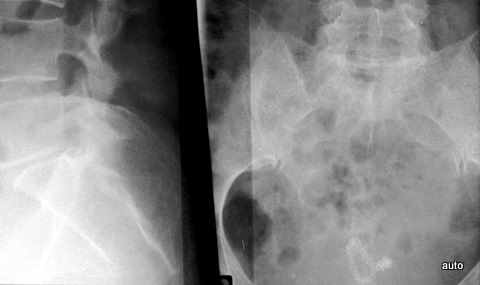
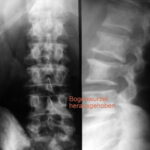
Fig. 11: Breast carcinoma. Important sign: “Loss of the arch root.” Right image after 1 year and 6 months. The progression shows that the blurring of the arch root L2 left (#) was indeed a true finding. Similarly, the contour loss of the vertebral body (small *) and the destruction of the transverse process were evident. Also, there was progressive destruction of Th12. Follow up 1 year and 6 months.
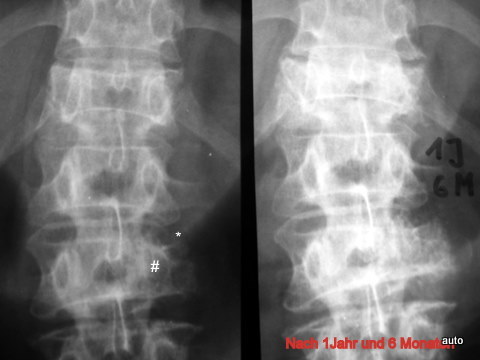
Fig. 11a: Disappearance of an arch root must be recognized. Likewise, unusual prominence of an arch root can occur in osteoblastic metastasis due to the deposition of osteoblastic tissue on the inner side of the cortex.
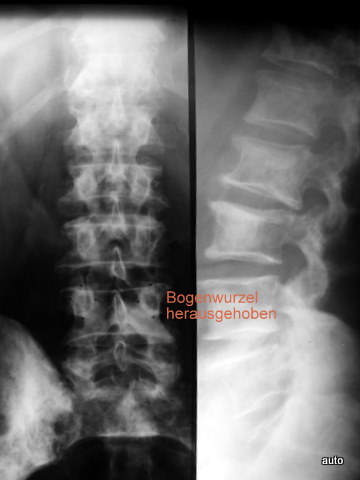
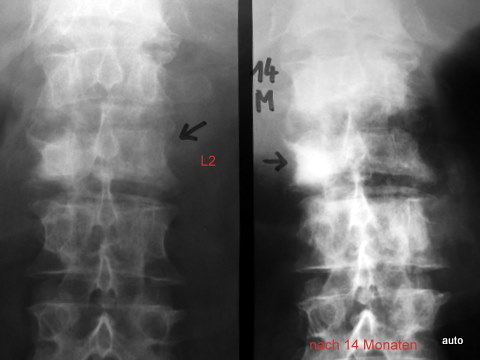
Fig. 11b: If this was not believed in Fig. 11a: An overlay of osteoblastic metastases in the vertebral body can make an arch root appear prominent (L2 right). The brighter depiction emphasizes this part of the arch. On the left at L2: Progressive lysis of the arch root in 14 months.
5. Imaging Section 3
Fig. 12: Lesions in the humerus? No cortical involvement. In the context of the rib lesion extending beyond the contour, the humerus finding is also highly suspicious for BM. Confirmation by scintigraphy.
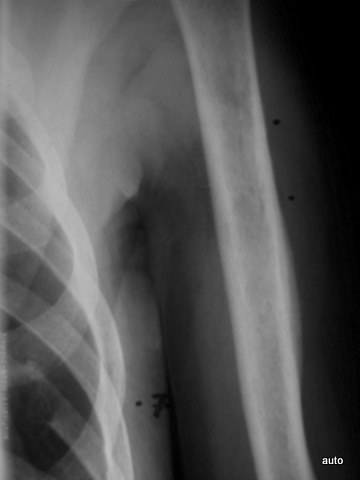
Fig. 13: Loss of a physiological contour at the lower edge of dorsal rib 9 should not be mistaken as a suspicious lysis.
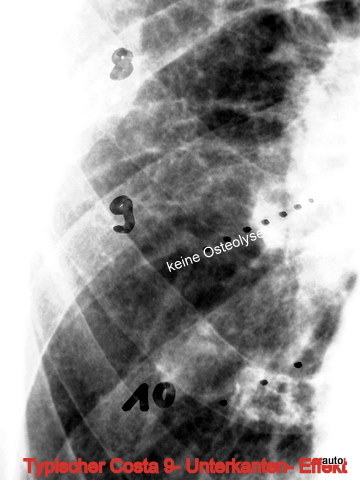
Fig. 14: Thyroid carcinoma. The loss of entire rib segments due to a metastasis with a large soft tissue component is often overlooked! Therefore, a systematic review of all ribs is essential during thoracic imaging. Brighten the image if needed! A rib is marked with a wire. Two ribs higher, there is destruction with a soft tissue tumor. One rib lower appears to be entirely missing (?).
It is still surprisingly difficult for beginners to make a diagnosis.
Now that the eye is trained, the next case becomes easier.
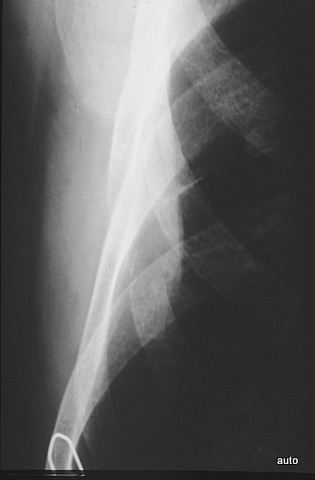
Fig. 14a: Classic case of metastatic rib destruction (thyroid carcinoma) with a soft tissue tumor. Severe clinical symptoms!
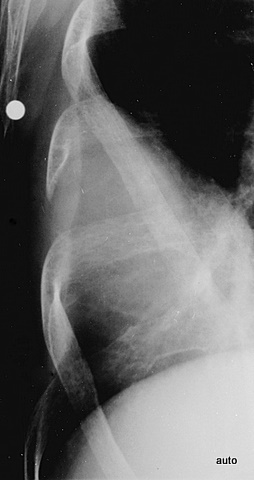
Fig. 15: Breast carcinoma. Shoulder pain. The marked sclerosis and structural loss of the 4th rib were missed during the initial examination.
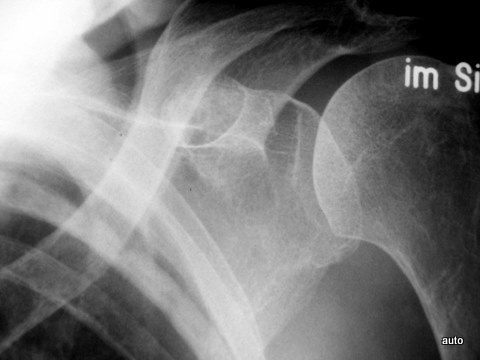
Fig. 15a: Same case. After a period of remission, recurrence of tumor progression after 4 years: Additional ribs affected (at least left side: 4, 6, 7, 10; right side: 4, 5, and 7 with predominantly osteoblastic metastases).
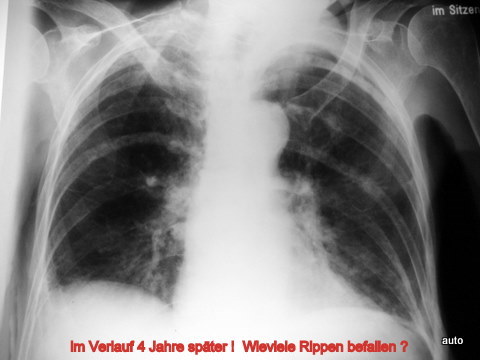
Fig. 16: Status post-epiphyseal displacement and dislocation of the femur during adolescence. At age 75, the patient developed gastric carcinoma with progressive metastases. Left hemipelvis (depicted twice, mirror-imaged) over a 7-month period. Retrospectively, the initial diagnosis of mixed-type metastases in the femur, and central pelvic region was difficult, later extending to the iliac crest and anterior pelvic ring.
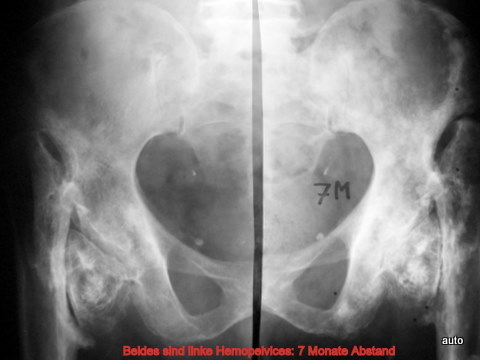
Fig. 16a: Breast carcinoma. Osteolyses predominantly in the central pelvic region on the right side. Loss of the right linea terminalis (arrow). Periosteal elevation noted.
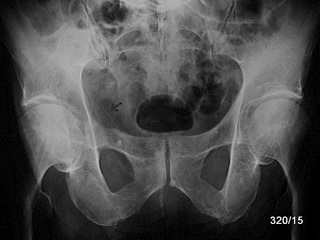
Fig. 16b: Bronchial carcinoma. Massive osteoblastic metastases causing expansion of the pelvic bones due to periosteal apposition.
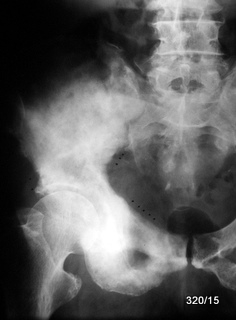
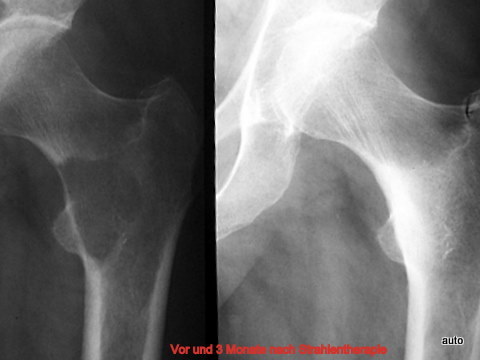
Fig. 17: Generalized bone metastases in breast carcinoma. Sharp border lines are unusual but can appear in metastatic lesions. After radiation therapy (4 weeks), rapid clinical improvement and significant sclerosis of the lysis were observed.
6. Summary and Didactic Notes
The radiological findings of bone metastases (BM) vary significantly depending on the primary tumor and the type of metastatic growth. The interpretation of imaging requires thorough knowledge of typical patterns and variations.
For accurate diagnosis, a systematic approach is essential, considering the full clinical context and utilizing advanced imaging modalities when necessary.